Species
Pyrrophycophyta
IUCN
NCBI
EOL Text
The dinoflagellates are an important group of phytoplankton (microscopic free-floating photosynthetic organisms) in both marine and freshwaters. They may occur as swimming, solitary cells or as nonmotile symbionts of various invertertebrates such as corals. Many are photosynthetic, but many others are not. Most of the photosynthetic species share certain types of pigment, including several pigments apparently found only in dinoflagellates. Numerous other aspects of the cell biology and genetics of dinoflagellates are unusual as well (reviewed in Hackett et al. 2004 and Wong and Kwok 2005). Some dinoflagellates produce toxins that may harm a wide variety of vertebrates and invertebrates (see Relevance). A large group of photosynthetic dinoflagellates are endosymbionts on which many corals and other invertebrates depend for their survival (see Associations).
| License | http://creativecommons.org/licenses/by-nc-sa/3.0/ |
| Rights holder/Author | Shapiro, Leo, Shapiro, Leo, EOL Rapid Response Team |
| Source | http://eolspecies.lifedesks.org/pages/15898 |
Based on recent molecular phylogenetic analyses by several researchers, it appears that the sister group to the dinoflagellates (i.e., the group with which the dinoflagellates share a most recent common evolutionary ancestor) is the Apicomplexa (Hackett et al. 2004), a group of protists that includes some taxa well known as parasites of humans and other animals. Among these familiar apicomplexans are Plasmodium (the cause of malaria), Cryptosporidium (the cause of cryptosporidiosis), Babesia (the cause of babesiosis), and Toxoplasma gondii (the cause of toxoplasmosis).
| License | http://creativecommons.org/licenses/by-nc-sa/3.0/ |
| Rights holder/Author | Shapiro, Leo, Shapiro, Leo, EOL Rapid Response Team |
| Source | http://eolspecies.lifedesks.org/pages/15898 |
Dinoflagellates are common organisms in all types of aquatic ecosystems. Roughly half of the species in the group are photosynthetic (Gaines and Elbrächter 1987), the other half is exclusively heterotrophic and feeds via osmotrophy and phagotrophy. As a consequence, they are prominent members of both the phytoplankton and the zooplankton of marine and freshwater ecosystems. They are also common in benthic environments and in sea ice.
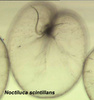
Noctiluca scintillans is a very large marine, planktonic, phagotrophic, athecate dinoflagellate that can cause pinkish red or greenish red tides, that is able to be bioluminescent, and that can contain green eukaryotic endosymbionts (Pedinomonas noctilucae). The shown specimen is from a temperate region (without endosymbionts) but the species can also be found in subtropical and tropical areas. © Mona Hoppenrath
In terms of morphology, dinoflagellates can be as varied and complex as any unicellular eukaryote. Complex organelles found in the group include structures reminiscent of a full-fledged vertebrate eye (but in a unicellular organism that lacks a brain), nematocysts comparable to those of cnidarians, and a bewildering array of plastid types in the photosynthetic forms. Dinoflagellates exist as plasmodia (i.e. multinucleate organisms), biflagellated cells, coccoid stages and even, in one small group, as cell arrays that approach multicellularity.
Genetically, dinoflagellates are also unique. The nucleus of a large majority of dinoflagellates (the so-called dinokaryotes) is so different from other eukaryotic nuclei that it has been given its own name, the dinokaryon. Dinokarya lack nucleosomes, and DNA content is orders of magnitude larger than that of other eukaryotic cells, for example those of humans. These dinokarya divide via a unique form of mitosis. Recent research is starting to show just how unique dinoflagellate genetic systems are. For example, gene products of all dinoflagellate nuclei (not only dinokarya) are processed in a unique way: a spliced leader is trans-spliced to all mRNA molecules. The genomes of plastids and mitochondria of the group are also unique: they are atomized (i.e. the genome is split into very small fragments), and gene content is much, much lower than that of comparable organelles in other organisms.
Approximately 4500 species assigned to more than 550 genera have been described, nearly three quarters of the genera and more than half of the species being fossil. Of the ca. 2000 living species, more than 1700 are marine and about 220 are from freshwater (Taylor et al. 2008). These numbers are sure to grow substantially in the future. Between the years 2000 and 2007 three new dinoflagellate families, 22 new genera, and 87 new species were described (Centre of Excellence for Dinophyte Taxonomy CEDiT). Recent molecular analyses have shown that there are large numbers of undescribed dinoflagellate species in environments like marine picoplankton (e.g. Moreira and López García 2002, Worden 2006) or as symbionts (‘zooxanthellae’) in many types of protists and invertebrates like corals (Coffroth and Santos 2005).
Practical Significance
Dinoflagellates are perhaps best known as causers of harmful algal blooms (webpages about this topic: ISSHA, WHOI, IOC). About 75-80% of toxic phytoplankton species are dinoflagellates (Cembella 2003), and they cause “red tides” that often kill fish and/or shellfish either directly, because of toxin production, or because of effects caused by large numbers of cells that clog animal gills, deplete oxygen, etc. (Smayda 1997). Dinoflagellate toxins are among the most potent biotoxins known. They often accumulate in shellfish or fish, and when these are eaten by humans they cause diseases like paralytic shellfish poisoning (PSP), neurotoxic shellfish poisoning (NSP), diarrheic shellfish poisoning (DSP) and ciguatera (Lehane and Lewis 2000). They also have been linked to major human health concerns, especially in estuarine environments (Pfiesteria). Some syndinians, notably Hematodinium, are parasites of economically-significant crustacean species.
The main ecological significance of dinoflagellates lies elsewhere, though. They are second only to diatoms as marine primary producers. As phagotrophic organisms they are also important components of the microbial loop in the oceans and help channel significant amounts of energy into planktonic food webs. As zooxanthellae, dinoflagellates have a pivotal role in the biology of reef-building corals.
| License | http://creativecommons.org/licenses/by-nc/3.0/ |
| Rights holder/Author | Mona Hoppenrath, Juan F. Saldarriaga, Tree of Life web project |
| Source | http://tolweb.org/Dinoflagellates/2445 |
Nash et al. (2008) discuss the many unusual features of the mitochondrial genome and their evolutionary implications.
| License | http://creativecommons.org/licenses/by-nc-sa/3.0/ |
| Rights holder/Author | Shapiro, Leo, Shapiro, Leo, EOL Rapid Response Team |
| Source | http://eolspecies.lifedesks.org/pages/15898 |
English-language taxonomic monographs covering large numbers of species are those by Steidinger and Williams (1970, Gulf of Mexico), Taylor (1976, Indian Ocean), Dodge (1982, British Isles), and Gómez (2003, Mediterranean). The taxonomy of extant and fossil species was unified for the first time by Fensome et al. (1993). A good summary of the biology of the group is presented in Hackett et al. (2004b). Papers concerned primarily with the evolution of the whole group include Taylor (2004), Saldarriaga et al. (2004) and Zhang et al. (2005). Two volumes edited by Spector (1984) and Taylor (1987) have brought together much general literature. Major reviews have been provided on particular aspects (e.g. Fensome et al. 1993: classification; Graneli and Turner 2006: biology of harmful species; Coffroth and Santos 2005: zooxanthellae).
| License | http://creativecommons.org/licenses/by-nc/3.0/ |
| Rights holder/Author | Mona Hoppenrath, Juan F. Saldarriaga, Tree of Life web project |
| Source | http://tolweb.org/Dinoflagellates/2445 |
Barcode of Life Data Systems (BOLD) Stats
Specimen Records:2222
Specimens with Sequences:1843
Specimens with Barcodes:1097
Species:156
Species With Barcodes:142
Public Records:1419
Public Species:119
Public BINs:14
Dinoflagellates have been studied and classified by botanists, zoologists and paleontologists, and this has resulted in differing taxonomic practices and dual (or even triple) classification schemes. Fensome et al. (1993) unified dinoflagellate classification.
As in every other group, molecular data have given much insight onto the phylogenetic history of dinoflagellates. However, this has not (yet) been translated into official renaming of higher taxonomic groups. The main reason for this is that most dinoflagellate phylogenetic trees have backbones that are poorly resolved, and so it is difficult to determine phylogenetic relationships of large groups to each other based on this kind of data alone (Daugbjerg et al. 2000, Saldarriaga et al. 2004). In dinoflagellates, the main value of molecular phylogenetic data have been to clarify in-group phylogenies, for example within groups like calciodinellids, pfiesteriaceans, polykrikoids or the genera Symbiodinium or Alexandrium, as well as to underline the differences between groupings of gymnodinoids. In addition, it has been gratifying to see that molecular data generally agree with classifications of gonyaulacalean genera based on tabulation. To a large degree this is also true in peridinioids, but here the situation is complicated by the ‘intromission’ of many prorocentralean, dinophysialean and even gymnodinialean groupings that form a so-called GPP group (gymnodinoids, peridinoids and prorocentroids).
One recent (and very welcome) trend has been the re-investigation of the type species of large, polyphyletic genera of gymnodinoid dinoflagellates like Gymnodinium, Gyrodinium, Amphidinium, with both ultrastructural and molecular methods. This has enabled a more phylogenetically-accurate circumscription of those large genera, and has caused a flood of descriptions of new gymnodinoid genera that are not particularly closely related to those types (e.g. Karenia, Karlodinium, Takayama, Togula, Prosoaulax, Apicoporus, Tovellia, Borghiella, Baldinia, Jadwigia). It should be noted, however, that Gymnodinium, Gyrodinium, Amphidinium, are formally still polyphyletic, they contain many species that have not been re-investigated recently or that have not yet been given new taxonomic placements. Recent papers have used the terms sensu lato and sensu stricto to distinguish between the polyphyletic and the newly-defined versions of these genera. In the case of Gymnodinium, even the ‘sensu stricto’ version of the genus is still paraphyletic, it has been shown that Polykrikos, Pheopolykrikos, Warnowia and Nematodinium are all decended from it (Hoppenrath and Leander 2007, and unpublished data). In this work, as in much of the primary literature, when there is reason to believe that a species is misclassified into a certain genus, that generic name is given inside apostrophes (e.g. ‘Gyrodinium’ dorsum, ‘Amphidinium’ longum, ‘Pheopolykrikos’ hartmanii).
| License | http://creativecommons.org/licenses/by-nc/3.0/ |
| Rights holder/Author | Mona Hoppenrath, Juan F. Saldarriaga, Tree of Life web project |
| Source | http://tolweb.org/Dinoflagellates/2445 |
The closest relatives of dinoflagellates are apicomplexans and ciliates. These three eukaryotic clades, together with the paraphyletic group that includes their ancestors, the protalveolates, form the so-called Alveolates (Cavalier-Smith 1991), one of the best-supported groupings that have emerged from the analysis of molecular phylogenetic data in eukaryotes (e.g. Fast et al. 2002, Cavalier-Smith and Chao 2004, and many others). Morphological data also strongly supports this clade (e.g. Taylor 2004). The closest relatives of alveolates are the heterokonts (also called stramenopiles). The relationship between alveolates and heterokonts is also very well supported with molecular data (e.g. Fast et al. 2001, Harper and Keeling 2003, Hackett et al. 2004a). Alveolates, heterokonts and a clade composed of cryptomonads and haptophytes have been proposed to constitute the so-called chromalveolates, one of the supergroups of eukaryotic diversity.
If the chromalveolate hypothesis is true (and if the dinoflagellate peridinin plastid is a vertical descendant of the original chromalveolate plastid), then the ancestor of all dinoflagellates was photosynthetic, and it contained the same type of plastids as the ancestor of all apicomplexans. The close relationship between dinoflagellates and apicomplexans, and the abundance of parasitic groups branching from the base of the dinoflagellate lineage (syndinians) argue furthermore for a parasitic (or perhaps mutualistic?) ancestor for the whole group. The recent discovery of a photosynthetic endosymbiont of corals, Chromera, with apicomplexan phylogenetic affinities strongly supports these two views (Moore et al. 2008). It was shown in that study that the apicomplexan apicoplast (a plastid remnant in that group) was derived from a red alga in the same endosymbiosis event that gave rise to the dinoflagellate peridinin plastid (Moore et al. 2008, Keeling 2008). This event could possibly have been the original chromalveolate endosymbiosis. Recent data also suggest that the nuclei of organisms from at least some of the early, non-photosynthetic branches of the dinoflagellate lineage, e.g. Perkinsus (Stelter et al. 2007, Matsuzaki et al. 2008) and Oxyrrhis (Slamovits and Keeling 2008) contain genes of a plastidial origin.
Whether the chromalveolate hypothesis turns out to be correct or not, at least the dinokaryotic non-photosynthetic dinoflagellates seem to have had photosynthetic ancestors: photosynthetic and non-photosynthetic forms always make mixed groups in phylogenetic trees, and since the typical dinoflagellate peridinin plastid is exceedingly unlikely to have originated more than once, a repeated loss of photosynthetic ability in the non-photosynthetic groups is a virtual certainty (Saldarriaga et al. 2001, Sánchez-Puerta et al. 2007). The presence of cryptic plastids in ostensibly non-photosyntetic forms (e.g. Sparmann et al. 2008) is significant in this regard. In some lineages, the peridinin-plastid was not only lost, but also replaced by either true plastids or plastid-like organelles with very different characteristics (see Plastids and Pyrenoids). The molecular mechanisms that enable this ‘plastidial promiscuity’ in dinoflagellates are poorly understood, but they are likely to involve signal sequences that tag nuclear-encoded proteins to peridinin-containing plastids somehow being re-directed to the new plastids. But the reasons why this happens in dinoflagellates and not in other groups with secondary plastids are entirely obscure.
| License | http://creativecommons.org/licenses/by-nc/3.0/ |
| Rights holder/Author | Mona Hoppenrath, Juan F. Saldarriaga, Tree of Life web project |
| Source | http://tolweb.org/Dinoflagellates/2445 |
Coral reef ecosystems are particularly sensitive to climate change. Since the 1980s, coral reef bleaching, caused by unusually high sea temperatures, has had devastating and widespread effects worldwide (Baker et al. 2008 and references therein). Environmental extremes, such as high or low temperatures or high irradiance, trigger a cacade of physiological and biochemical changes that lead to eventual cellular damage in the dinoflagellate symbionts and/or their coral hosts, and can lead to the expulsion of symbionts and the eventual breakdown of the symbiosis (Lesser 2004, 2006; Baker et al. 2008 and references therein). The loss of zooxanthellae (and/or a reduction in their pigment concentrations) as a result of this process is known as “bleaching”. In extreme cases, bleaching leads to the visible paling of the host organism, as the yellow-brown pigmentation of the symbionts is lost (Baker et al. 2008).
These episodes of mass coral bleaching and mortality have raised concerns about the long-term survival of coral reef ecosystems. There is increasing evidence that under "normal" conditions dinoflagellate communities in coral reefs are in flux, with species densities and species composition shifting in response to numerous factors including changes in environmental conditions, such as warming of the seas. Some researchers are hopeful that mass bleaching events, which involve large-scale losses of endosymbiotic zooxanthellae, are simply an extreme example of a "normal" transition in the dinoflagellate community composition and that given time some reef recovery is possible (Baker 2003 and references therein). (Some researchers have even argued that bleaching is an adaptive response to extreme environmental changes that allows corals to rapidly change their dinoflagellate associates to species better suited to the new environmental conditions.) Most ecological communities are quite resilient, but as is is often the case with extreme environmental perturbations, the question of whether long-term persistence of coral reefs is possible turns largely on questions of scale: Is the change too rapid for corals and their dinoflagellate associates to adapt? Might persistence be possible on a large geographic scale even if local extinctions are inevitable? If recovery is possible, how long might it take and how similar would the recovered reef systems be to those that preceded them?
| License | http://creativecommons.org/licenses/by-nc-sa/3.0/ |
| Rights holder/Author | Shapiro, Leo, Shapiro, Leo, EOL Rapid Response Team |
| Source | http://eolspecies.lifedesks.org/pages/15898 |